Is Oncodona for you?
This test is recommended if:
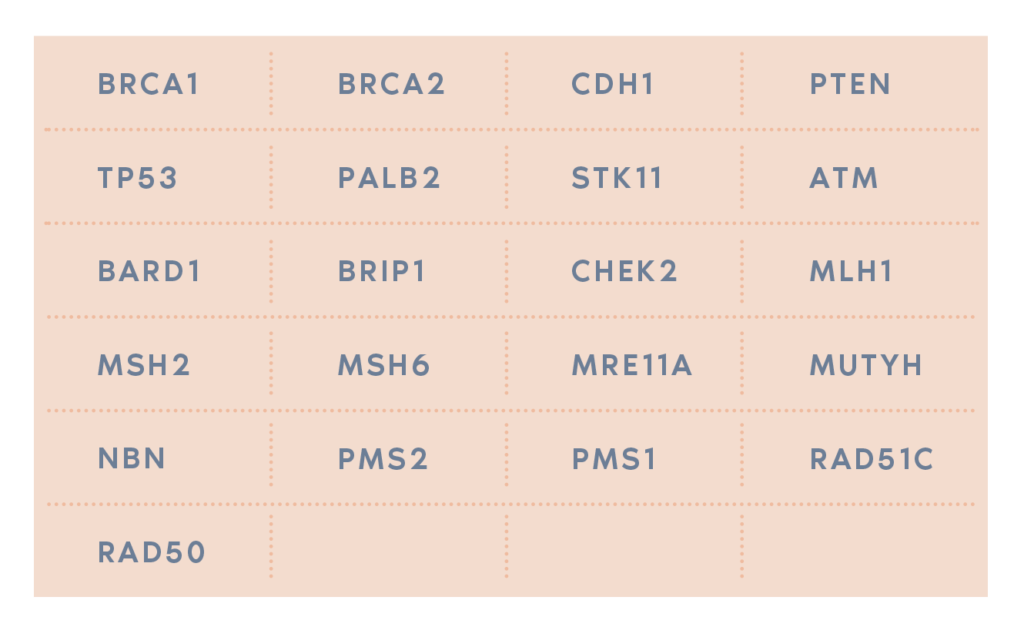
- You’ve been diagnosed with breast cancer and BRCA1 and BRCA2 negative genetic test. Oncodona can find a mutation in up to 4% of women.
- A woman in your family has been diagnosed with breast and ovarian cancer.
- You just want to know if you have any risk of developing breast and ovarian cancer.